Assertion : The α-hydrogen atom in carbonyl compounds is less acidic.
Reason : The anion formed after the loss of α -hydrogen atom is resonance stabilised.
• The carbon next to any functional group (here carbonyl group) is called the α-carbon and the H group attached to it is called the α-hydrogen atom. The reason is a wrong statement because the α-hydrogens in carbonyl compounds are generally acidic in nature and in chemical reactions there will be an easy loss of these in forms of H+ ions.
• The main reason behind this is electron withdrawing (attracting) effect of the carbonyl group which is powerful enough to remove α-hydrogens by any base (as H is acidic).
• Another fact also contributes to this acidity which is the resonance stabilisation of the anion (i.e. the conjugate base of the acid) formed (called enolate ion) after the removal of the α-hydrogens which is stated in the reason, which would have been explained the assertion if it was correct.
• 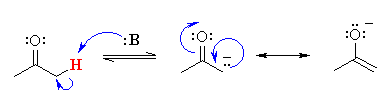
enolate ion