Assertion : Aromatic aldehydes and formaldehyde undergo Cannizaro reaction.
Reason : Aromatic aldehydes are almost as reactive as formaldehyde.
• Cannizaro is actually a type of disproportionation reaction (a reaction involving both oxidation and reduction). And in this case of aldehydes which do not have any α-H atoms and therefore undergoes self oxidation in the presence of concentrated alkali solutions (NaOH or KOH) to form acid and reduction to form alcohols.
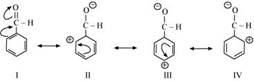
• Aromatic aldehydes also do not have α-H so they also undergo this reaction.
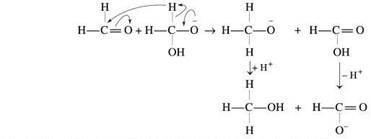
• But aromatic aldehydes cannot be almost reactive as the aliphatic aldehydes like formaldehyde so the reason statement is wrong because the aromatic aldehydes are usually very stabilised by the more resonance stabilization of the aromatic ring attached with it which making its carbonyl carbon less acidic or electrophillic unlike formaldehyde where there is no such ring present so the carbonyl carbons will be more acidic or electrophillic(electron loving nature) .