Assertion : Aldehydes and ketones, both react with Tollen’s reagent to form silver mirror.
Reason : Both, aldehydes and ketones contain a carbonyl group.
• Reaction with Tollen’s reagent (which is actually the ammoniacal silver nitrate solution) is actually is an oxidation reaction.
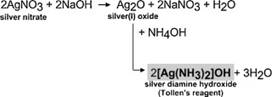
• But only the aldehydes respond to Tollen’s reagent which oxidises aldehydes to carboxylic acids and simultaneously aldehydes reduce the Ag+ ions of this reagent to metallic silver and a brightly appeared silver mirror is formed on the sides of the test tube(silver mirror test )
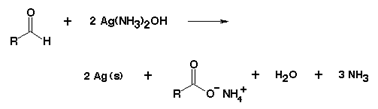
• Ketones do not give reactions with Tollen’s reagent, therefore the assertion statement is wrong because they are not oxidised by weak oxidising agents as they are less acidic than aldehydes (absence of any α-H atoms hence inability to reduce silver ions).
• And the reason statement cannot explain the reactions of tollen’s reagent because in spite of having carbonyl group, aldehydes and ketones reacts to it differently.