An aromatic compound ‘A’ (Molecular formula C8H8O) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound ‘B’ on treatment with iodine and sodium hydroxide solution. Compound ‘A’ does not give Tollen’s or Fehling’s test. On drastic oxidation with potassium permanganate it forms a carboxylic acid ‘C’ (Molecular formula C7H6O2), which is also formed along with the yellow compound in the above reaction. Identify A, B and C and write all the reactions involved.
compound A is obviously is a ketone because it gives positive 2,4- DNP and positive iodoform test but does not respond to Tollen’s or Felling’s solution ( not aldehyde).
• With the molecular formula of C8H8O and D.B.E. calculation of : 8 (carbon no.s) –(8/2) (hydrogens no./2) +0 (no nitrogen) +1 = 5, therefore it has 5 unsaturations in forms of the ring or double bonds or both.
• Hence the structure of A should be :
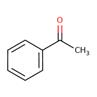
i.e. acetophenone, justified by the 4 double bonds, 1 ring, and one carbonyl carbon.
• It gives positive 2,4-DNP test :

• It gives a positive iodoform test:

‘B’ (yellow ppt)
Hence the yellow compound formed on iodoform reaction is CHI3.
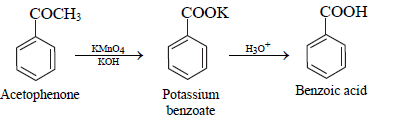
• On drastic oxidation with potassium permanganate, it forms a carboxylic acid ‘C’ which is actually benzoic acid which is formed from acetophenone.
 structure of ‘C’
structure of ‘C’
The reaction is as follows :