Write down functional isomers of a carbonyl compound with molecular formula C3H6O. Which isomer will react faster with HCN and why? Explain the mechanism of the reaction also. Will the reaction lead to the completion with the conversion of whole reactant into product at reaction conditions? If a strong acid is added to the reaction mixture what will be the effect on concentration of the product and why?
If we want to write the structural isomers of the molecular formula C3H6O, we have to calculate the double bond equivalent (D.B.E.) i.e. the degree or no. Of unsaturation this molecular formula has.
• D.B.E. = C – (![]() ) + (
) + (![]() ) + 1 where, C=the no. Of carbon atoms, H=the no. Of hydrogen atoms, N= the no. Of nitrogen atoms.
) + 1 where, C=the no. Of carbon atoms, H=the no. Of hydrogen atoms, N= the no. Of nitrogen atoms.
• For C3H6O , D.B.E. will be = 3 – (![]() ) + 0 + 1= 3 -3+ 1 = 1 ; hence it will have only one unsaturation in all of its isomers.( Unsaturation stands for double or ripple bonds and cyclic rings also.)
) + 0 + 1= 3 -3+ 1 = 1 ; hence it will have only one unsaturation in all of its isomers.( Unsaturation stands for double or ripple bonds and cyclic rings also.)
• Therefore the structures are,
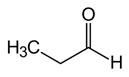 ,
,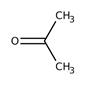 ,
,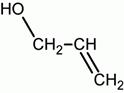 ,
, 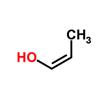 , ,
, ,
propanal Propanone (acetone) allyl alcohol 1-propenol
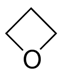 ,
, 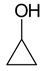 ,
, 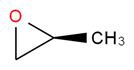 ,
, 
Oxacyclobutane cyclopropanol propylene oxide methyl vinyl ether.
• Among all these isomers only propanal (aldehyde) and acetone will react with HCN; which is a type of nucleophilic addition reaction. (HCN being a nucleophile i.e. love for nucleus or positive charge) .
• But the corresponding aldehyde will give a faster and more productive reaction as it is more active than its ketonic counterpart due to-
(I)Electronic factor: the presence of an α-H which leads to resonance stabilisation (by hyperconjugation) on the carbonyl carbon atom which makes it more electrophillic and more prone towards nucleophilic addition and
(II). Steric factor: The steric hindrance in acetone due to two alkyl group on each side of the carbonyl carbon which makes it more difficult for the nucleophile to attack.
Mechanism of the reaction as follows :
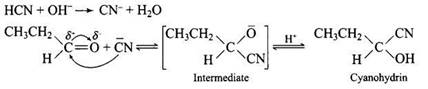
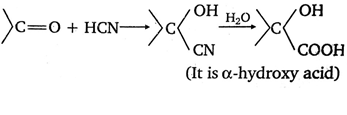
HCN has no lone pair of electrons thus cannot act as a nucleophile alone. However if the reaction is carried out in presence of a base (OH - ); it yields cyanide ions CN- which acts as a nuclephile and the reaction proceeds and cyanohydrins, a new compound is formed.
• At these reaction conditions, the nucleophilic addition reaction is reversible hence , the reaction will not lead to the completion of the reaction with the conversion of the whole reactant into the product at reaction conditions, that will be partial.
• If a strong acid will be added to this reaction mixture , the cyanohydrins will undergo hydrolysis which will lead to formation of the reaction lead to the completion with the conversion of the whole reactant into product at reaction conditions α hydroxy butanoic acid (CH3CH2CH(OH)COOH) which will be produced in more concentrated state as the reaction is irreversible as more stable product is formed.