When liquid ‘A’ is treated with a freshly prepared ammoniacal silver nitrate solution, it gives bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogensulphite. Liquid ‘B’ also forms a white crystalline solid with sodium hydrogensulphite but it does not give test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
• Obviously the liquid ‘A’ is the aldehyde among the two because it gives a positive silver mirror test also reacts with sodium hydrogen sulphite to give a white crystalline solid (the bisulphite addition compound of the corresponding aldehyde A)
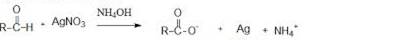
‘A’ Silver mirror
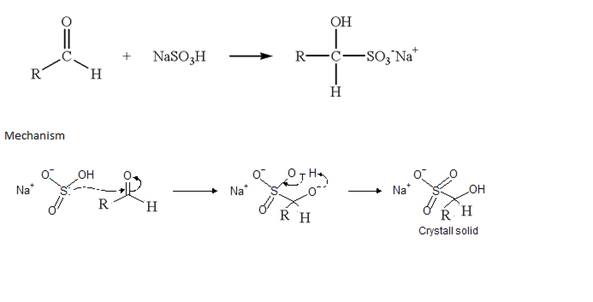
• Ketones also respond to this reaction with sodium hydrogen sulphite and form the same type crystalline addition product.
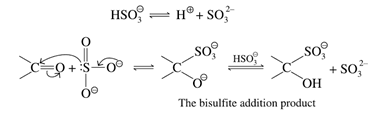
• But when it comes to tollen’s reagents ketone do not give this test because ketones do not get oxidised by mild oxidising agents for the absence of an α-H atom.
• Hence tollen’s reagent test is the clear distinction between aldehydes and ketones. Therefore in the given problem liquid, ‘A’ must be an aldehyde because it gives a positive tollen’s test and the other liquid ‘B’ must be a ketone as though it does not give tollen’s test but it reacts with NaHSO3 and forms the addition crystalline product.