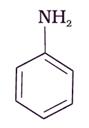
The correct increasing order of basic strength for the following compounds is _________.
(I)
(II)
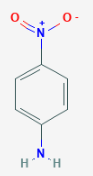
(III)
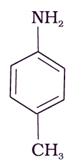
• p-toluedene (III)  is the strongest base among the 3 given compounds because the presence of −CH3 group in the p position, which has electron-donating (+I effect) that increases the electron density on the N-atom atom and make the electron pairs more available for protonation.
is the strongest base among the 3 given compounds because the presence of −CH3 group in the p position, which has electron-donating (+I effect) that increases the electron density on the N-atom atom and make the electron pairs more available for protonation.
• In case of I and II aniline is moe basic than III p-nitroaniline; but aniline is less basic than III p- toluedene.
• p-nitroaniline there is a nitro group in the ‘para’ position of the benzene ring which is an electron-withdrawing group and involves the lone pair on N atom in resonance thus decreasing its ability to donate unshared electron pairs to acid.

• In case of aniline the conjugation  is not as effective as p-nitroaniline so it is a better base.
is not as effective as p-nitroaniline so it is a better base.