In the nitration of benzene using a mixture of conc. H2SO4 and conc. HNO3, the species which initiates the reaction is __________.
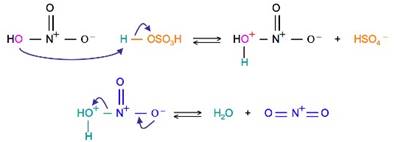
at first, this step takes place because there is a requirement for stronger electrophile and for that nitric acid has to be activated for nitration of benzene.
• Sulphuric activates nitric acid and produces a strong electrophile nitronium ion NO2+, its electrophilicity tends to attack the benzene ring and electrophillic substitution takes place by producing nitrobenzene.
•
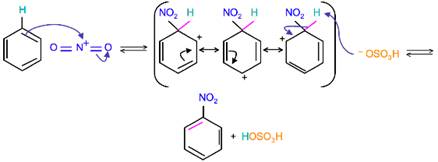
The other 3 species (i) NO2(ii) NO+ and (iv) NO2– cannot be formed during the activation of nitric acid.
1