Which of the following should be most volatile?
(I) CH3CH2CH2NH2
(II) (CH3)3N
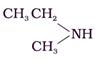
(III)
(IV) CH3CH2CH3
• Volatility of a compound depends upon its bonding; strong bonding or interactions between molecules lead to more bulkiness and therefore less volatility.
• In case of CH3CH2CH3 there is no sufficient eletronegativity of C to form a hydrogen bond, which is likely to be the case for amines because they are associated with hydrogen bonding of N-H..........N type; this significant intermolecular hydrogen bonding causes amines to have higher boiling points than comparable hydrocarbons.
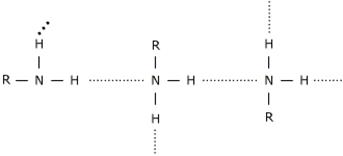
Hence CH3CH2CH3 will the most volatile of them all.
• But, (II) (CH3)3N and (III)![]() will be more volatile than (I) CH3CH2CH2NH2 because in case of secondary and tertiary amines, hydrogen bonding is not as possible as the primary amines as there is less N-H bonds present in secondary amine
will be more volatile than (I) CH3CH2CH2NH2 because in case of secondary and tertiary amines, hydrogen bonding is not as possible as the primary amines as there is less N-H bonds present in secondary amine ![]() and in the tertiary amine (CH3)3N there is no such N-H bond is present.
and in the tertiary amine (CH3)3N there is no such N-H bond is present.