The molar heat capacity for the process shown in the figure is

Let us consider the figure given below :
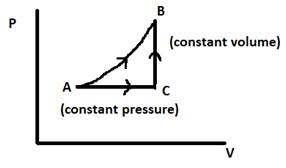
We consider the process AB to be composed of two processes, AC(at constant pressure) and CB(at constant volume), such that AB = AC + CB. C is the molar heat capacity of AB, Cp is the molar heat capacity of AC(constant pressure) and Cv is the molar heat capacity of CB(constant volume).
From the first law of thermodynamics, we know that Q = U + W … (i),
Where Q = heat supplied, U = change in internal energy, W = work done on the system.
Since the change in internal energy is independent of the path, it will have the same value for paths AB and ACB.
Hence, UAB = UACB … (ii), where UAB = change in internal energy for path AB, UACB = change in internal energy for path ACB.
Now, the work done by a process is given by the area under the PV diagram. We can clearly see that the area under process AB is greater than the sum of areas under processes AC and CB.
Hence, WAB > WACB … (iii), where WAB = work done for process following path AB, WACB = work done for process following path ACB.
Adding (ii) and (iii), we get :
UAB + WAB > UACB + WACB … (iv)
But, from the first law of thermodynamics, Q = U + W, where Q = heat supplied, U = change in internal energy, W = work done on the system.
Hence, we get, QAB > QACB … (v),
We know, QAB = nCdT, QAC = nCpdT, QCB = nCvdT,
where QAB = heat supplied in process AB
QAC = heat supplied in process AC
QCB = heat supplied in process CB
n = number of moles
C = molar heat capacity
Cp = molar heat capacity at constant pressure
Cv = molar heat capacity at constant volume
dT = change in temperature.
Also, QACB = QAC + QCB … (vi), where
QACB = heat supplied in process ACB
QAC = heat supplied in process AC
QCB = heat supplied in process CB
Hence, from (v), we get,
nCdT > nCpdT + nCvdT
Dividing by ndT on both sides :
C > Cp + Cv => C > Cv (option c)