Let ΔWa and ΔWb be the work done by the systems A and B respectively in the previous question.
We know that for any given state, the slope of the p-V diagram of an adiabatic process is -γP/V, while that of an isothermal process is -P/V.
Hence, the slope of an adiabatic process is more.
Now, the area under the curve of a p-V diagram gives the work done.
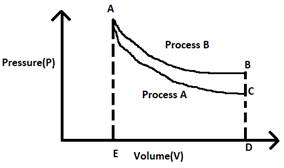
From this diagram, we can see that that area under the curve of process A(ACDE) which represents the adiabatic process with greater slope is less than that of the area under the curve of process B(ABDE), which represents the isotherm.
Hence, we can conclude that ΔWa < ΔWb
1