Consider the processes A and B shown in figure. It is possible that
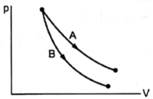
From the graph, we can see that the slope of process B is steeper than that of process A.
Now in an isothermal process, under constant temperature, Pressure(P) x Volume(V) = constant … (i) (according to Boyle’s law)
Differentiating on both sides:
PdV + VdP = 0.
slope = ![]() =
= ![]() … (ii)
… (ii)
For an adiabatic process,
PVᵞ = constant
Where,
1