A vessel containing one mole of a monatomic ideal gas (molecular weight = 20 g mol–1) is moving on a floor at a speed of 50 ms–1. The vessel is stopped suddenly. Assuming that the mechanical energy lost has gone into the internal energy of the gas, find the rise in its temperature.
Given:
number of moles, n = 1
Specific heat at constant temperature, Cv(monoatomic gas) = 3R/2 = 12.471 J/mol/K
R = universal gas constant = 8.314 J/mol/K
Initial velocity(vi) = 50 m/s
Final velocity(vf) = 0
Molecular weight(m) = 20 g/mol = 0.02 kg/mol
Formula Used:
i. Change in internal energy(dU) = nCvdT,
Where,
n is the number of the moles of the gas,
Cv is the heat capacity at the constant volume
dT = rise in temperature.
ii. Mechanical energy lost, 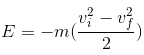
Where,
m is the molecular weight of the gas in kg
vi is the initial velocity,
vf is the final velocity,
equating equation (i) and (ii), we get
Putting the values in the above equation, we get
![]()
=> dT = rise in temperature ~ 2 K (Answer).