An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation p = kV. Show that the molar heat capacity of the gas for the process is given by 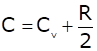 .
.
Given:
P = kV …
Where,
P = pressure,
V = volume,
k = constant.
Formula used:
Equation of state of ideal gas:
PV = nRT = constant … (ii)
Where,
n is the number of moles of the gas,
R is the gas constant,
T is the temperature,
P = pressure, V2
T = temperature.
From (i), multiplying by dV on both sides:
PdV = kVdV.
Integrating from V = V1 to V2, we get
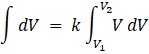
= ![]() with lower limit V1 and upper limit V2
with lower limit V1 and upper limit V2
= ![]()
Now, we know, PV = nRT - equation of state,
Where P = pressure, V = volume, n = number of moles, R = universal gas constant, T = temperature
Hence we can write, V1 = nRT1/P1. Since P1 = kV1, this becomes:
kV12 = nRT1. Similarly, KV22 = nRT2,
P1, V1, T1 - Pressure, volume, temperature of first gas
P2, V2, T2 - Pressure, volume, temperature of second gas
Therefore, subsituting, the above equation becomes:
= ![]() =
= ![]() … (iii)
… (iii)
Now, ![]() =>
=> ![]() (since P = kV) … (iv)
(since P = kV) … (iv)
But Q = U + ഽPdV (first law of thermodynamics), where Q = heat, U = change in internal energy, W = total work done = ഽPdV
=> nCdT = nCvdT + (nR/2)dT
(since Q = nCdT and U = nCvdT)
=> C = Cv + nR/2 (proved),
Where
n = number of moles,
C = specific heat capacity,
Cv = specific heat capacity at constant volume,
R = universal gas constant,
dT = rise in temperature.