Two ideal gases have the same value of CP/CV = γ. What will be the value of this ratio for a mixture of the two gases in the ratio 1: 2?
We know Cp/Cv=γ, R=Cp-Cv,
where the molar heat capacity C, at constant pressure, is represented by Cp, at constant volume, the molar heat capacity C is represented by Cv and R is the universal gas constant.
Now,
![]()
For the first ideal gas,
![]()
![]()
Where Cp1 and CV1 is the molar heat capacity at constant pressure and constant volume
Similarly, for the second ideal gas
![]()
![]()
Where Cp2 and CV2 is the molar heat capacity at constant pressure and constant volume
Given,
![]()
i.e
dU1=nCV1dT
dU2=2nCV2Dt
When gas is mixed,
![]()
![]()
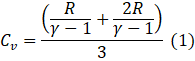
Also,
![]()
From (1) and (2)
![]()
1