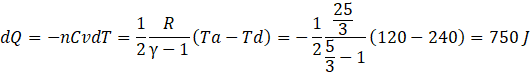Half mole of an ideal gas (γ =5/3) is taken through the cycle abcda as shown in figure. Take ![]() .
.
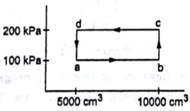
(a) Find the temperature of the gas in the states a, b, c and d.
(b) Find the amount of heat supplied in the processes ab and bc.
(c) Find the amount of heat liberated in the processes cd and da.
Given, n=1/2, γ =5/3, R=25/3 J/Kmol
a) By ideal gas equation,
![]() where P, V and T are the pressure, volume and absolute temperature; n is the number of moles of gas; R is the ideal gas constant.
where P, V and T are the pressure, volume and absolute temperature; n is the number of moles of gas; R is the ideal gas constant.
Here temperature at a,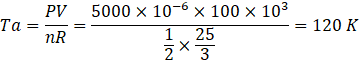
Here temperature at b,
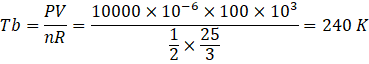
Here temperature at c,
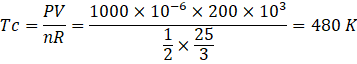
Here temperature at d,
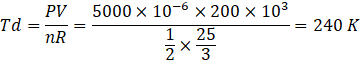
b) Here ab is an isobaric process where heat supplied dQ can be expressed as
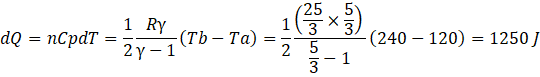
Here bc is an isochoric process where heat supplied dQ is
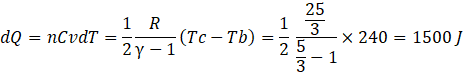
c) Heat liberated in cd, isobaric process dQ is
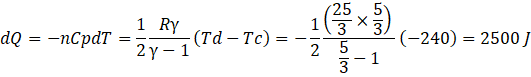
Heat liberated in da, isochoric process dQ is