Consider a given sample of an ideal gas (CP/CV = γ) having initial pressure P0 and volume V0.
(a) The gas is isothermally taken to a pressure P0/2 and from there adiabatically to a pressure P0/4. Find the final volume.
(b) The gas is brought back to its initial state. It is adiabatically taken to a pressure P0/2 and from there isothermally to a pressure P0/4. Find the final volume.
Given CP/CV = γ, initial pressure P1=P0 and initial volume V1= V0
a) Since the gas is isothermally taken to pressure P2=P0/2
∴ ![]()
![]()
![]()
Let P3=P0/4 and V3 be the pressure and volume after adiabatic compression.
Then,
PVγ = const.
I.e.![]()
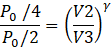
Substituting value of V2=2V0
![]()
(b)Here P1=P0, P2=P0/2 and the process is adiabatic. Let V1=V0 be the initial volume and V2 be the volume after process.
Then,
PVγ = const.
I.e.![]()
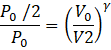
I.e. ![]()
Let P3= P0/4 and V3 be the pressure and volume after isothermal process Then,
![]()
![]()
![]()