Three samples A, B and C of the same gas (γ = 1.5) have equal volumes and temperatures. The volume of each sample is doubled, the process being isothermal for A, adiabatic for B and isobaric for C. If the final pressure is equal for the three samples, find the ratio of the initial pressures.
Let VA, VB, VC be the volume of three gases and TA, TB, TCbe the temperature of A, B, C gas
Given, TA=TB=TC, VA=VB=VC
Here A is undergoing an isothermal process, where V1= VA, V2=2VA
Let P1![]() and P2
and P2![]() be the initial and final pressures,
be the initial and final pressures,
Then,
![]()
![]()
![]()
Here B is adiabatic,
PVγ = const, where V1= VB, V2=2VB
Let P1![]() and P2
and P2![]() be the initial and final pressures,
be the initial and final pressures,
I.e.![]()
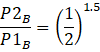
![]()
Here C is isobaric ,the pressure remains constant and equal to ![]()
Now, as the final pressures are equal for all the gases
![]()
![]() , ratio of the initial pressures
, ratio of the initial pressures