The half-life of a radioisotope is 10 h. Find the total number of disintegrations in the tenth hour measured from a time when the activity was 1 Ci.
We know that,
![]()
![]()
Where,
A= Activity of the substance
A0 = Initial activity
t = time
λ = decay constant
Given,
Half-life = 10h, A0=1 Ci
Activity after 9 hours ![]()
= 0.536 Ci
Number of atoms left after 9 hours ![]()
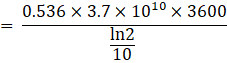
![]()
Activity after 10 hours = 0.5 Ci [As 10h is half-life]
Number of atoms left after 10 hours ![]()
![]()
![]()
Hence, net disintegrations at the 10th hour = (1.03×1015-9.60×1014)
= 6.92×1013 atoms
1