A vessel of volume 125 cm3 contains tritium (3H, t1/2 = 12.3 y) at 500 kPa and 300 K. Calculate the activity of the gas.
Given: Pressure P= 500000 Pa= 5 atm
Volume V= 0.125L
Temperature T= 300K
Assuming the gas to be ideal, according to ideal gas equation,
![]() (R be universal gas constant equal to 0.082atmLmol-1K-1, P is the pressure, V is the volume, T is the temperature)
(R be universal gas constant equal to 0.082atmLmol-1K-1, P is the pressure, V is the volume, T is the temperature)
So, ![]()
![]()
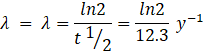
Activity, ![]()
![]()
![]()
1