A hydrogen atom contains one proton and one electron. It may be assumed that the electron revolves in a circle of radius 0.53 angstrom (1 angstrom = 10–19 m and is abbreviated as ![]() ) with the proton at the center. The hydrogen atom is said to be in the ground sate in this case. Find the magnitude of the electric force between the proton and the electron of a hydrogen atom in its ground state.
) with the proton at the center. The hydrogen atom is said to be in the ground sate in this case. Find the magnitude of the electric force between the proton and the electron of a hydrogen atom in its ground state.
Given:
Magnitude of charge on both proton and electron, ![]()
The radius of the circular orbit, ![]()
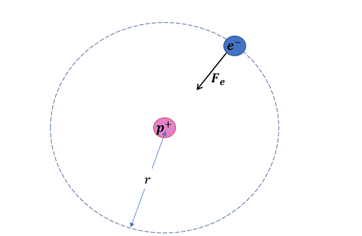
The radius of the circular orbit is the distance between the electron and the proton.
___________________________________________________
Formula used:
By Coulomb’s law, the electric force is given by:
![]()
Where ϵ0 is the permittivity of free space
q1 and q2 are the magnitude of charges
r is the distance of separation between the charges
(Here, ![]() )
)
___________________________________________________
The magnitude of electric force is given by:
![]()
Putting the values in the above formula, we get
![]()