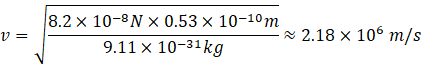Find the speed of the electron in the ground state of a hydrogen atom. The description of ground state is given in the previous problem.
Given:
Magnitude of charge on both proton and electron, ![]()
The radius of the circular orbit, ![]()
Mass of electron , ![]()
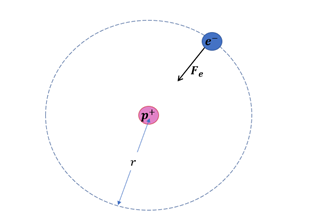
The radius of the circular orbit is the distance between the electron and the proton.
___________________________________________________
Formula used:
By Coulomb’s law, the electric force is given by:
![]()
Where ϵ0 is the permittivity of free space
q1 and q2 are the magnitude of charges
r is the distance of separation between the charges
(Here, ![]() )
)
___________________________________________________
The magnitude of electric force is given by:
![]()
![]()
___________________________________________________
Formula used:
Centripetal force is given by
![]()
Where m is the mass if the object, v is the speed of the object and r is the radius of the circular path.
___________________________________________________
Let Fc be the centripetal force on the electron.
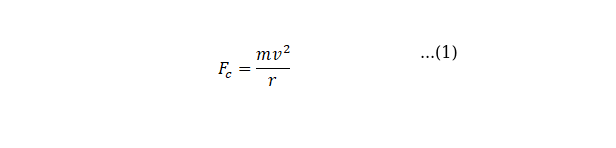
Now, the electric force acts as centripetal force.
Hence,
![]()
Substituting (1) and rearranging, we get
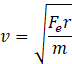
Putting the values in the above formula, we get