What will be the molarity of a solution, which contains 5.85 g of NaCl(s) per 500 mL?
The molarity of a solution is defined as the number of moles of a solute dissolved in 1 litre of a solution. The given solute is NaCl and its molar mass is 58.44 ~ 58.5 g. Therefore, a 1M solution of NaCl will contain 58.5g of NaCl dissolved in 1 litre of solvent.
The molarity of the given NaCl solution is given by the formula
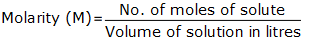
= ![]() = 0.1/0.5 = 0.2M or 0.2 mol L-1.
= 0.1/0.5 = 0.2M or 0.2 mol L-1.
The correct answer is (iii).
4