The reactant which is entirely consumed in reaction is known as limiting reagent. In the reaction 2A + 4B → 3C + 4D, when 5 moles of A react with 6 moles of B, then
(i) which is the limiting reagent?
B. calculate the amount of C formed?
The equation in terms of moles of reactants and products is given as
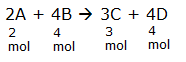
Considering the definition of limiting reagent, we consider two cases where each reactant is completely consumed.
Case (I): Reactant A is completely consumed.
5 moles of A and 6 moles of B are given.
If 2 mol of A gives 3 mol of C, then 5 mol of A will give 3 x 5/2 = 7.5 mol of C.
Case (II): Reactant B is completely consumed.
5 moles of A and 6 moles of B are given.
If 4 mol of B gives 3 mol of C, then 6 mol of B gives 6 x 3/4 = 4.5 mol of C.
If reactant B is completely consumed, lesser amount of product C is formed, hence the limiting reagent is B.
(i) The limiting reagent is B
(ii) The amount of C formed is 4.5 moles.