Match the following physical quantities with units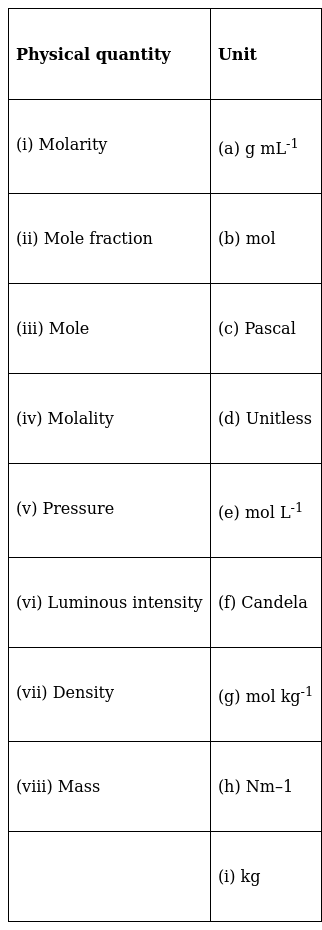
The units of the physical quantities are as follows:
(i) – (e)
As we all know that Molarity is defined as;
![]()
So, SI unit of Molarity is mole/litre.
(ii) – (d)
As we all know that Mole fraction is defined as given below;
Mole fraction of A = ![]()
And, both of the nominator and denominator and have same SI unit. Thus, the SI unit of mole fraction is unitless.
(iii) – (b)
SI unit of Mole is simply mol.
(iv) – (g)
Molality = ![]()
So, from the above formula we can conclude that the SI unit of Molality is molekg-1.
(v) – (c)
SI unit of Pressure is Pascal.
Although, it is defined as given below;
Pressure = ![]() .
.
Although, the SI unit of the above formula is ![]() . Which is also known as Pascal.
. Which is also known as Pascal.
(vi) – (f)
In photometry, luminous intensity is a measure of the wavelength-weighted power emitted by a light source in a particular direction per unit solid angle, based on the luminous function, a standardized model of the sensitivity of the human eye.
The SI unit of luminous intensity is the candela(cd).
(vii) – (a)
The formula of density is given below;
![]()
So, from the above formula, we can conclude that SI unit of Density is gml-1.
(viii) – (i)
As we all know that mass is measured in Kg and the SI unit of Mass is Kg.