A box contains some identical red coloured balls, labelled as A, each weighing 2 grams. Another box contains identical blue coloured balls, labelled as B, each weighing 5 grams. Consider the combinations AB, AB2, A2B and A2B3 and show that law of multiple proportions is applicable.
The law of multiple proportions states that if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
The given data is tabulated as follows:
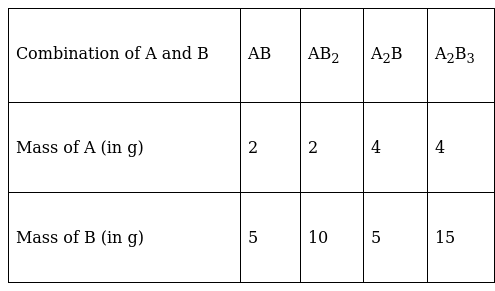
Consider the first two examples AB and AB2 where the mass of A is fixed and the mass of B is not. The ratio of masses of B with the fixed mass of A is 5:10 or 1:2 which is a simple whole number ratio. Here the law of multiple proportions is applicable.
In the second two examples, A2B and A2B3, the ratio of masses of B with the fixed mass of A is 5:15 or 1:3, which is also a simple whole number ratio.