Explain why does the enthalpy change of a reaction remain unchanged even when a catalyst is used in the reaction.
OR
With the help of an example explain what is meant by pseudo first order reaction.
The role of a catalyst is to provide an alternative path by forming an activated complex with lower activation energy without changing the enthalpy of reaction.
In the presence of a catalyst, the Gibbs free energy does not change as the energy difference between reactant and product remains the same.
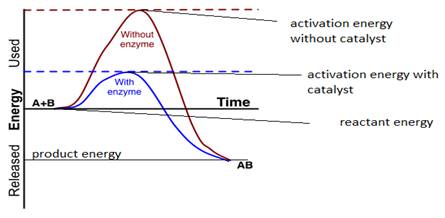
A reference can be taken from the above graph that final product AB energy and the reactants (A and B) energies are the same in the absence and the presence of catalyst remains the same in the presence or absence of a catalyst.
OR
A pseudo-first-order reaction is a reaction that is truly second order but can be approximated to be first-order under special circumstances.
For Example, Hydrolysis of .01 mole of ethyl acetate in the presence of 10 moles of water, the reaction at first glance appears to be the first-order reaction but since the concentration of water does not change much in comparison to ethyl acetate the order of reaction becomes pseudo-first-order reaction.
For a pseudo-first-order reaction, the reactant with a large amount of concentration is ignored.
As the change in its concentration is negligible with comparison to another component in respect with time.
Hence, the rate only gets dependent upon the reactant in small quantity.