Which of the following graphs represents exothermic reaction?
(a)
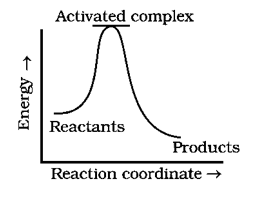
(b)

(c)
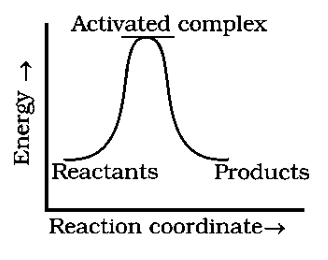
For an exothermic reaction the energy of reactants should be greater than that of the product.
For graph (a) follows the statement, and when the reaction goes in the forward direction the difference in energy of reactant and product is released in the form of heat so the reaction is an exothermic reaction.
For Graph (b) the energy of the product is greater than that of a reactant. Hence, the reaction is endothermic in nature for the forward direction.
In graph (c) the energy of product and reactant is same. Hence, it is neither endothermic nor exothermic in nature.
2