According to Maxwell Boltzmann distributon of energy, __________.
The Maxwell Boltzmann distribution of energy can be further studied from the graph below,
Which shows a decrease in most probable Kinetic Energy (peak of the graph) due to the increase in collisions among the molecules of gas with an increase in temperature making option (iv) correct.
There is also an increase in the fraction of molecules having energy greater than EA and most probable Kinetic Energy due to the increase in temperature T, as the molecules of gas get thermally excited due to the increase in temperature making option (ii) correct.
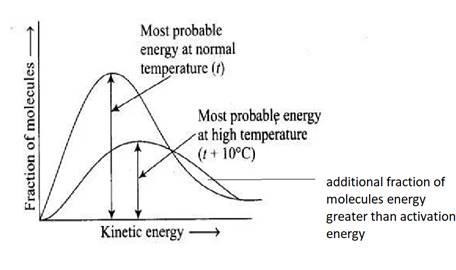
Maxwell Boltzmann distribution of energy.
1