In the graph showing Maxwell Boltzman distribution of energy, ___________.
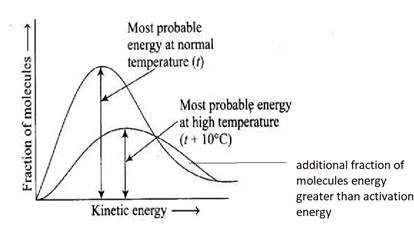
The area under the graph remains constant since the total energy of all molecules present in a sample remains 1 for all time even on increasing temperature making a statement (i) correct.
The most probable energy decrease due to the collision between molecules but the number of molecules with activation energy EA increases due to the increase in temperature.
The temperature curve shifts right and get broadens at higher temperature due to the involvement of additional molecules whose energy goes above activation energy due to an increase in temperature and decrease in the most probable energy of the molecule making a statement (iv) also correct.
1