For a general reaction A → B, plot of concentration of A vs time is given in Fig. 4.3. Answer the following question on the basis of this graph.
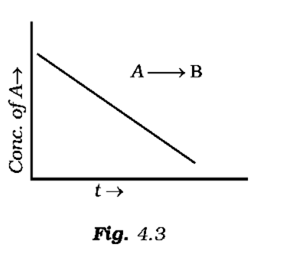
(i) What is the order of the reaction?
(ii) What is the slope of the curve?
(iii) What are the units of rate constant?
(i) It is a zero order reaction as the graph is satisfying the equation [A] = [A0] – kt.
(ii) The slope of the curve is the negative of the rate constant that is denoted by –k.
(iii) Unit of rate constant is Ms-1 or mol L-1s-1.
1