The empirical formula and molecular mass of a compound are CH2O and 180 g respectively. What will be the molecular formula of the compound?
The empirical formula of a compound represents the simplest whole number ratio of various atoms present in the molecule, while the molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
The empirical formula mass of the compound is calculated to be 1x12 + 1x2 + 1x16 = 30.
The molecular mass of the compound is 180.
‘n’ is the factor to be multiplied with the empirical formula to give the molecular formula and it is given by
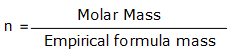
n = 180/30 = 6
Molecular formula = n→Empirical formula) = 6→CH2O)
The molecular formula of the compound is C6H12O6.
The correct answer is →iii).