Match the correct ionisation enthalpies and electron gain enthalpies of the following elements.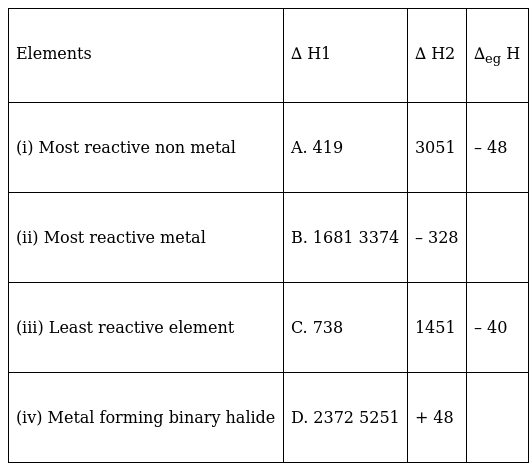
(i) Most reactive non metal is B
REASON: It has the highest negative electron gain enthalpy.
(ii) Most reactive metal =A
REASON: It has the lowest negative electron gain enthalpy as well as lowest first ionisation energy.
(iii) Least reactive element=D
REASON:D is the least reactive element as it has an extremely high first and second ionisation enthalpy.
(iv) Metal forming binary halide=C
REASON: it has low first ionisation enthalpy than second but the different is not much.
1