Explain the shape of BrF5.
In BrF5 the central atom is bromine which has the hybridization sp3d2.
Br atom has 7 valence electrons out of which 5 are used to make pair with the F atoms and two are used to make lone pair of electrons.
The lone pair and bond pair repel each other. Thus, the shape is square pyramidal.
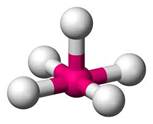
The white balls are F atoms and the pink ball is Br atom.
1