Write Lewis structure of the following compounds and show formal charge on each atom.
HNO0 , NO2, H2SO4 Charge given is wrong
The formal charge is calculated:

![]()
The formal charge on the oxygen with single bond =![]()
The formal charge on the oxygen with double bond![]()
The formal charge on nitrogen=![]()
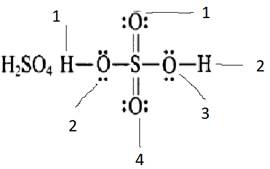
The formal charge on oxygen 1 and 4 =![]()
The formal charge on oxygen 2 and 3 =![]()
The formal charge on hydrogen 1 and 2 =![]()
The formal charge on sulfur =![]()
1