Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.

The hybridization of Carbon 1 is sp, carbon 2 is sp, carbon 3 sp2, carbon 4 is sp3 and carbon 5 is sp2.
The triple bond has 2 pie bonds and one sigma bond.
Each double bond has one sigma and one pie bond.
Every single bond is a sigma bond.
Thus, the total number of sigma bonds is 11 and pie bonds are 4.
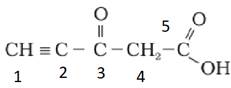
1