Match the items given in Column I with examples given in Column II.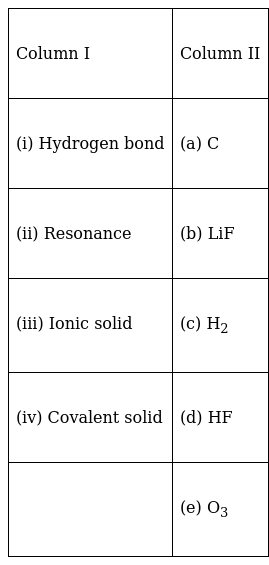
(i) ![]() (d);
(d);
the HF compound has hydrogen bonding due to a difference in the electronegativity of hydrogen and fluorine.
(ii) ![]() (e);
(e);
Ozone molecules have resonating structures.

(iii) ![]() (b);
(b);
LiF is an ionic compound Li donates one electron and fluorine accepts one to complete its octet.
(iv) ![]() (a);
(a);
Carbon is a covalent solid due to its sharing of electron and catenation property it forms agiant molecule with 3-d network.
1