Which of the following compounds would undergo SN1 reaction faster and why?
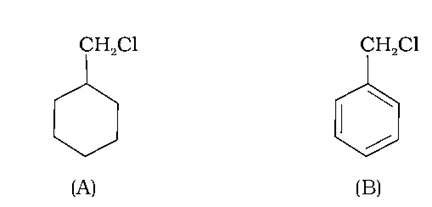
(B)-Benzyl chloride would undergo faster SN1 than (A)-Chloromethyl cyclohexane as the carbocation formed from benzyl chloride is resonance stabilized. In the case of chloromethyl cyclohexane the carbocation formed is primary. Primary carbocations are not favored by SN1 reactions as a result it will not undergo SN1 reaction as fast as the benzyl chloride.
1