Match the elements given in Column I with the properties mentioned in Column II.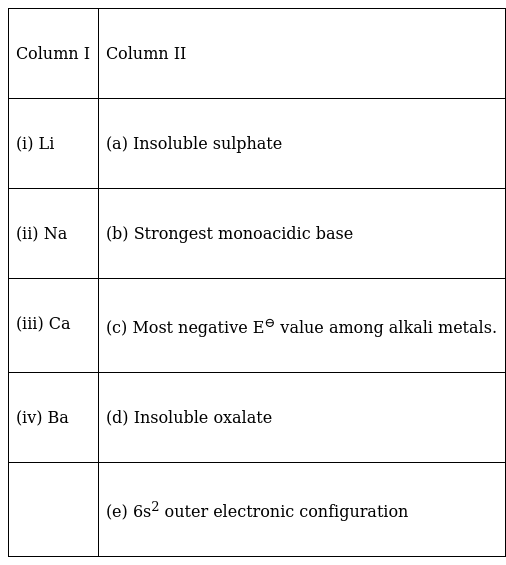
(i) → (c)
As Li has the most negative E⊖ value due to the high hydration energy.
(ii) → (b)
As Alkali metals are more acidic than the given alkaline earth metals, while Li base is covalent in nature.
(iii) → (d)
Calcium oxalate is insoluble in water
(iv) → (a)
Due to the high hydration energy.
(iv) → (e)
Electronic configuration of Ba is 1s22s22p63s23p63d104s24p64d105s25p66s2.
1