Match the compounds given in Column I with their uses mentioned in Column II.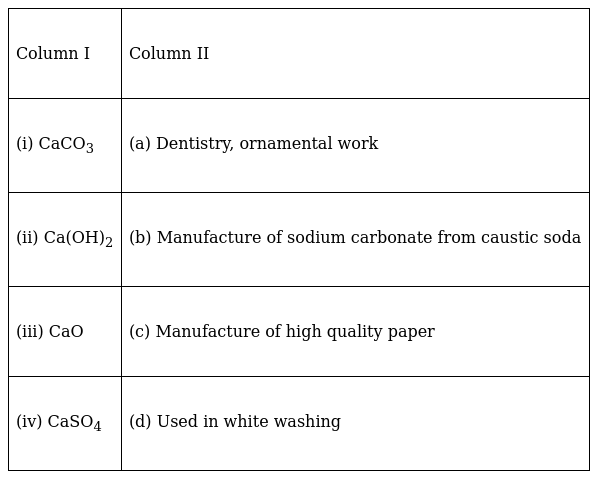
(i) → (c)
Calcium carbonate used in manufacturing high quality paper because the paper and cardboard industries use lime-based coating pigments and filters such as GCC(Ground Calcium Carbonate). GCC is made from concentrated and fine-ground calcium carbonate and used to make fine paper, cardboard packaging and pulp-based paper.
(ii) → (d)
Calcium Hydroxide is used in white washing because for white washing walls as it slowly reacts with CO2in air to form a thin layer of Calcium Carbonate on the walls. The layer of Calcium Carbonate gives a shiny finish to the walls.
Ca(OH)2 + CO2→ CaCO3 + H2O
(iii) → (b)
CaO is used in manufacturing of sodium carbonate from caustic soda.
Above process is also known as Solvay Process. This reaction occurs in two steps.
First step,
NaCl + CO2 + NH3 + H2O → NaHCO3 + NH4Cl(l)
First Ammonia converts into Sodium Hydrogen Carbonate and Ammonium Chloride.
Second Step,
2NaCl + CaCO3→ Na2CO3 + CaCl2
So, in the next or second step, there is the formation of Sodium Carbonate.
(iv) → (a)
CaSO4 is used in Dentistry, Ornamental work because CaSO4with adequate quantity of water, it forms a plastic mass that sets into a hard solid.