Match the elements given in Column I with the colour they impart to the flame given in Column II.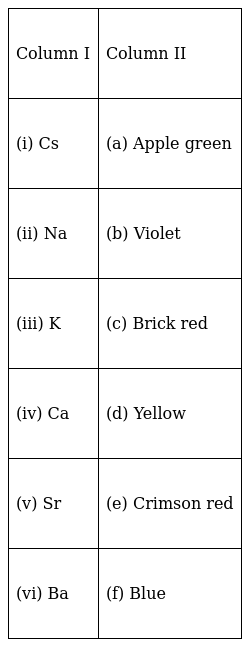
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
(i) → (f)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
So, Cs imparts blue colour.
(ii) → (d)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
Thus, Na imparts yellow colour.
(iii) → (b)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
Thus, Potassium imparts violet colour.
(iv) → (c)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
Thus, Ca imparts brick red colour.
(v) → (e)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
Thus, Sr imparts crimson red colour.
(vi) → (a)
The colour of the flame imparted depends on the energy required for electron excitation and deexcitation. This happens because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum.
Thus, Barium imparts apple green colour.