What is the role of benzoyl peroxide in addition polymerisation of alkenes? Explain its mode of action with the help of an example.
Benzoyl peroxide helps to generate free radical in chain initiation step which is the first step of the reaction in Addition polymerization reaction. Thus it acts as a free radical generating initiator catalyst.
Addition polymerization reaction occurs in three steps.
i) Chain initiation step ii) Chain propagating step iii) Chain terminating step
i) Chain initiation step:
In this step, a mixture of ethene with small amount of benzoyl peroxide is heated or is exposed to light. In the presence of heat or light, the O-O linkage in benzoyl peroxide breaks to generate two molecules of benzoyl free radical and finally to two molecules of phenyl free radical.
Later, this phenyl free radical is captured by the ∏-bond of Ethene molecule to generate a larger free radical.
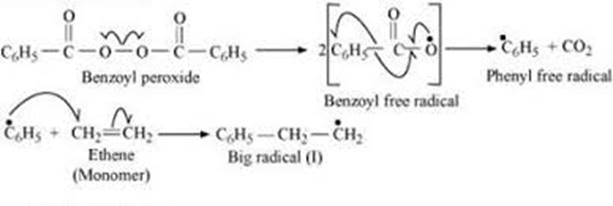
ii) Chain propagating step:
This larger free radical ![]() generated in chain initiation step further reacts with the second molecule of ethane to form another larger size free radical.
generated in chain initiation step further reacts with the second molecule of ethane to form another larger size free radical.
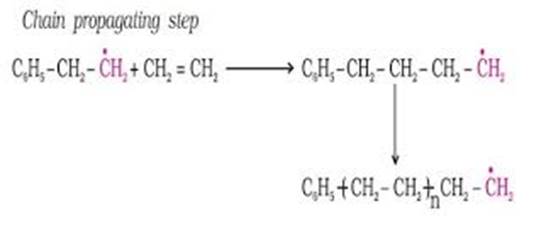
This propagation step continues to form larger and larger free radical which is comprised of ‘n' number of Ethene molecules, where n is a very large number.
iii) Chain terminating step:
Since the various free radicals are formed in chain propagation step, eventually, they collide with each other producing a final molecule. Chain termination is a random process, polythene will be made up of chains of different lengths.
To terminate the chain propagation step, chain-terminating step can be done in different ways,otherwise, we cannot get the desired polymerized product polythene.
One of the modes for the termination of the chain is given below:
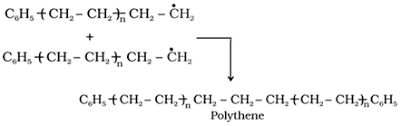
Thus we get our desired polymer polythene.