Match the polymer of column I with correct monomer of column II.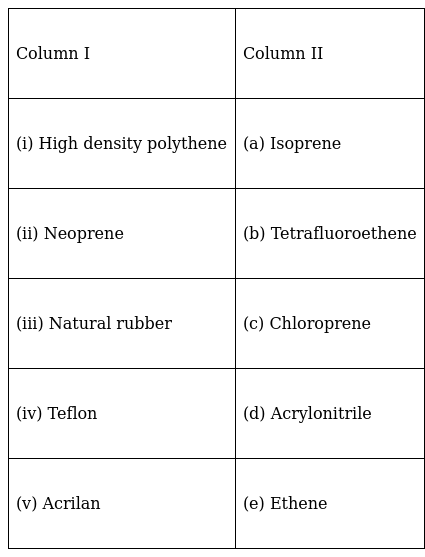
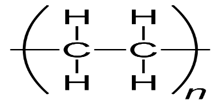
(i) High-density polythene(HDP) – (e) Ethene.
Explanation- Polythene is the polymer formed by the addition polymerization of ethene. There are two types of polythenes namely- High-density polythene(HDP) and Low-density polythene(LDP).
(ii) Neoprene – (c) Chloroprene.
Explanation- Neoprene (also known as polychloropropene) is a polymer formed from chloroprene which is 2-chloro-1,3-butadiene through polymerization by the production of free radicals in the intermediate stages of the reaction. It is thus called free radical polymerization.
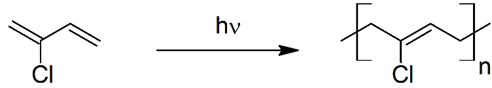
Note:-We know hν stands for sunlight and as there is sunlight it is obvious that the reaction will proceed via formation of free radicals.
(iii) Natural Rubber – (a) Isoprene
Rubber is a natural polymer and possess elastic properties. Natural rubber consists of a linear polymer of 2-methyl-1,3-butadiene which is commonly known as isoprene. It is also called cis-1,4-polyisoprene.
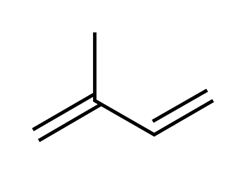
This is isoprene.
(iv) Teflon – (b) Tetrafluoroethene
Teflon is prepared by the polymerization of Tetrafluoroethene(PTFE) molecules. It is done so by heating PTFE molecules under high pressure and in presence of ammonium peroxo sulphate [(![]()
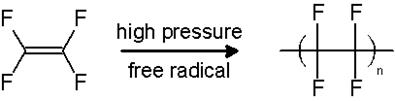
Tetrafluoroethene Teflon
(v) Acrilan – (d) Acrylonitrile
Acrilan is formed by the addition polymerization of acrylonitrile in presence of a peroxide catalyst. Acrilan is better known as Polyacrylonitrile(PAN) or orlon.
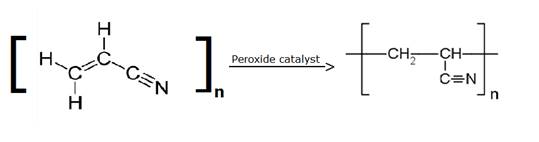
Acrylonitrile Polyacrylonitrile
Note:- Some polymers have their name starting with ‘Poly’ are examples of addition polymers. Example- Polythene, Poly tetrafluoro ethylene(Teflon), Polyacrylonitrile(Orlon), Polyisoprene ,Polyvinyl chloride(PVC) ,etc.