Match the polymers given in Column I with their chemical names given in Column II.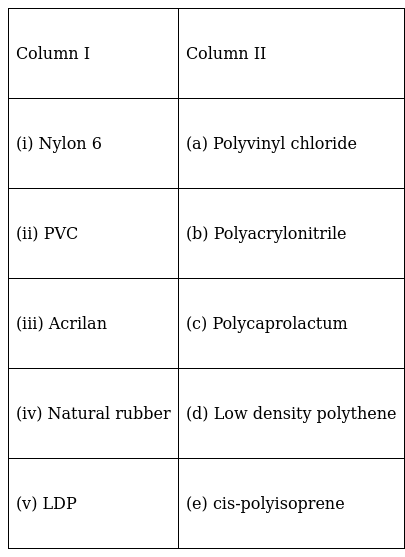
(i) Nylon-6 – (c) Polycaprolactum
For the preparation of Nylon-6(or Perlon), the first caprolactum is prepared from cyclohexane

Then caprolactum is heated with traces of water and on continued heating polymerization occurs and Nylon-6 is produced.

(ii) PVC – (a) Polyvinyl Chloride
When multiple molecules of vinyl chloride (![]() is heated in an inert solvent in the presence of dibenzoyl peroxide then PVC is obtained.
is heated in an inert solvent in the presence of dibenzoyl peroxide then PVC is obtained.

(iii) Acrilan – (b) Polyacrylonitrile
Polyacrylonitrile(PAN) or Acrilan is a thermoplastic (which becomes soft when heated and hard when cooled and can be moulded into shapes) hard material having high melting point. It is prepared by polymerization of acrylonitrile. Its another commercial name is Orlon.

(iv) Natural rubber – (e) cis-polyisoprene
As we have discussed earlier that natural rubber consists of a linear polymer of 2-methyl-1,3-butadiene which is commonly known as isoprene. It is also called cis-1,4-polyisoprene or simply cis-polyisoprene.

Thus, Above is the structure of isoprene

(v)LDP - (d) Low-density polythene
We know as discussed earlier that, there are two types of polythenes namely- High-density polythene(HDP) and Low-density polythene(LDP).