Match the polymers given in Column I with their commercial names given in Column II.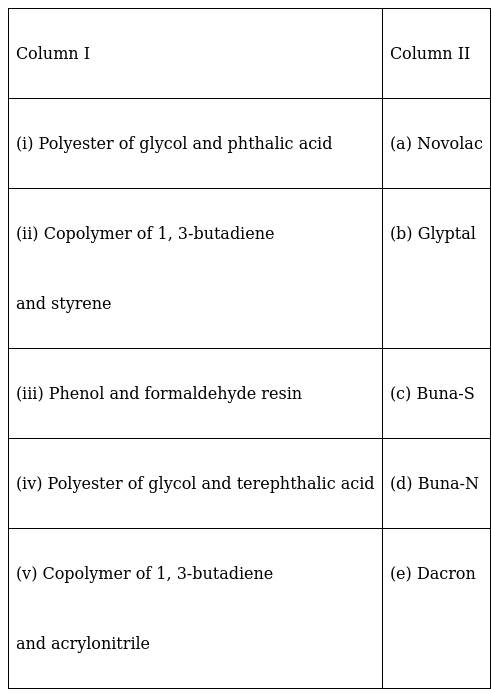
(i)Polyester of glycol and pthalic acid – (b) Glyptal
Polyester means polymers having the ester linkage 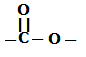 . So we get to know that glyptal has ester linkage in it.
. So we get to know that glyptal has ester linkage in it.
Now glyptal is a polyester obtained by the condensation of ethylene glycol and pthalic acid. It involves the removal of two simple H2O molecules.
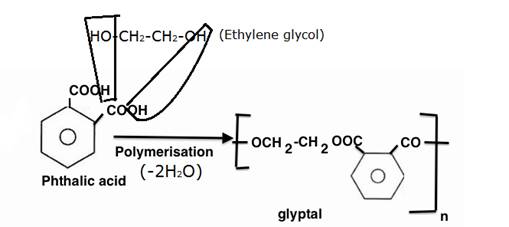
This is done so to show diagrammatically how the two water molecules are eliminated thereby forming glyptal.
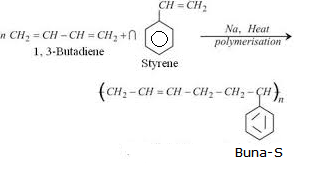
(ii) The Copolymer of 1,3-butadiene and styrene – (c) Buna-S
Buna-S is prepared from two monomers, 1,3-butadiene(![]() ) and Styrene(
) and Styrene(![]() ).
).
Note:
In the name Buna-S, Bu stands for butadiene, na for sodium which acts as polymerizing agent and S stands for styrene.
(iii) Phenol and formaldehyde resin – (a) Novolac
Novolac is prepared from phenol and formaldehyde. It is thus known as phenol-formaldehyde resin commonly as Bakelite. 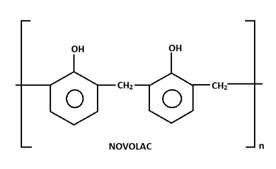
Note:
Novolac on heating with formaldehyde(HCHO) undergoes cross-linking to form an infusible mass called bakelite.
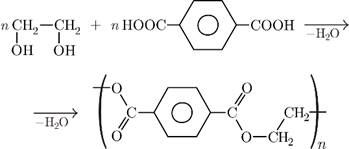
(iv) The Polyester of glycol and terepthalic acid – (e) Dacron
Teylene also known as Dacron is prepared by the condensation polymerization reaction of two monomers – Ethylene glycol and Terepthalic acid. Condensation reaction involves the loss of a simple molecule like NH3, H2O, HCl, etc(here, H2O is removed).
(v) Copolymer of 1,3-butadiene and acrylonitrile – (d) Buna-N
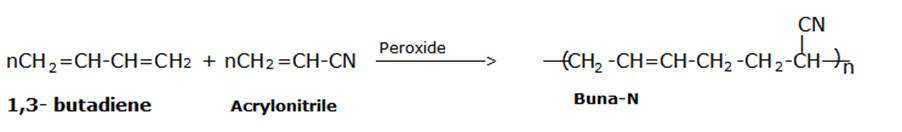
Buna-N is obtained by the copolymerization of 1,3-butadiene and acrylonitrile in the presence of a peroxide catalyst.