Differentiate between rubbers and plastics on the basis of intermolecular forces.
Rubber is a natural polymer that is capable of returning to its original length, shape, and size after the removal of forces (stretching or deformation). Rubber is a common example of an elastomer.
Rubber is the product when the molecules of isoprene undergo polymerization. The chains of polyisoprene are held together by weak van der Waals forces. Rubber has a coiled structure. 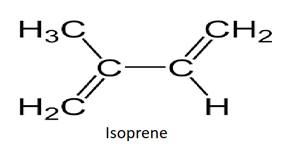
Plastics are synthetic or semi-synthetic polymers of organic compounds which possess intermediate intermolecular forces of attraction. The forces are intermediate in between those of elastomers and fibers.
It has a linear, structure that can be molded but cannot regain its original shape after stretching is over.
They are neither too weak or too strong and have no cross-linkage between the chain.
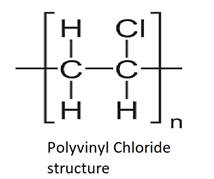
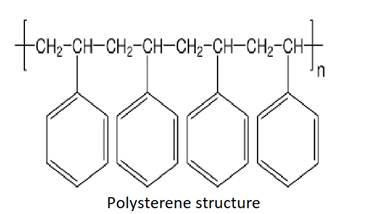
Few examples of plastics are given below with the structure: