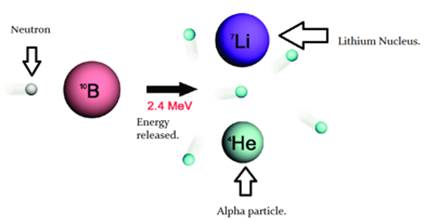
When a boron nucleus (10B) is bombarded by a neutron, an α-particle is emitted. Which nucleus will be formed as a result?
- When boron having atomic no.5 and atomic mass 10 is bombarded with neutron, the atomic no. decreases by 2 i.e.5-2=3 and the atomic mass decreases by 2 times of atomic no. i.e.4. Thus Lithium with atomic no.3 and mass number 6.
1