A sample contains a mixture of 110Ag and 108Ag isotopes each having an activity of 8.0 × 108 disintegrations per second. 108Ag is known to have larger half-life than 110Ag. The activity A is measured as a function of time and the following data are obtained.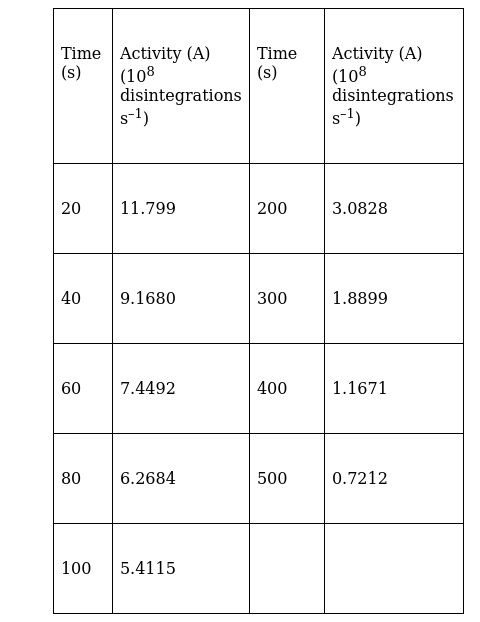
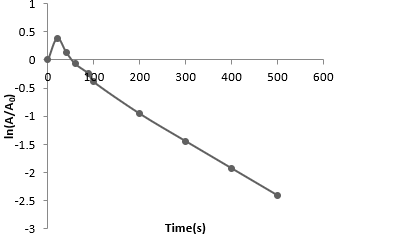
(a) Plot ln(A/A0) versus time.
(b) See that for large values of time, the plot is nearly linear. Deduce the half-life of 108Ag from this portion of the plot.
(c) Use the half-life of 108Ag to calculate the activity corresponding to 110Ag in the first 50 s.
(d) Plot ln(A/A0) versus time for 110Ag for the first 50 s.
(e) Find the half-life of 108Ag.
At time t=0, A˳= 8.0 × 108 dis/s
(a) ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
(b) For large values of time, the value of λ will be the slope negative of the slope of the curve.
∴ λ = 0.028s-1
So, t1/2= 24.4 s
(c) ![]()
(d) ![]()
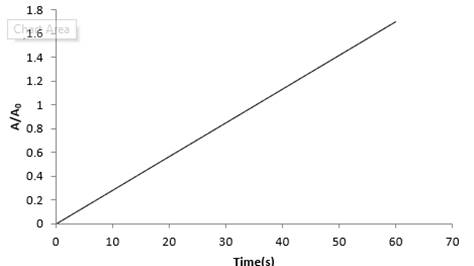
(e) The half-life of 108Ag from the graph is 144s.