Unlike dry cell, the mercury cell has a constant cell potential throughout its useful life. Why?
The following diagram represents a mercury cell.
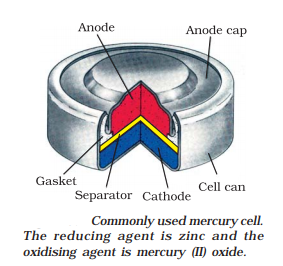
The reactions take place inside a mercury cell are as follows:
Anode: Zn(Hg) + 2OH–→ ZnO(s) + H2O + 2e–
Cathode: HgO + H2O + 2e–→ Hg(l) + 2OH–
Galvanic cell equation: Zn(Hg) + HgO(s) → ZnO(s) + Hg(l)
The above overall reaction contains no ions whose concentration can change over time. So, the cell potential remains constant throughout the mercury cell life.
1