Match the terms given in Column I with the items given in Column II.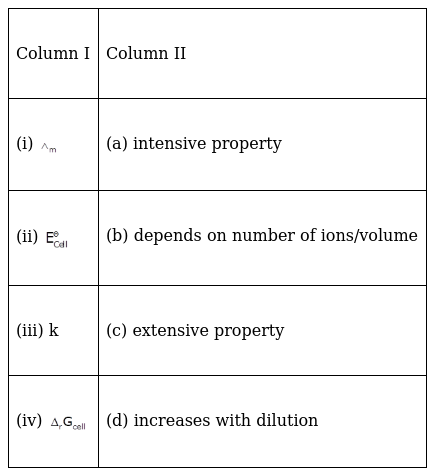
(i) Λm → (d) increases with dilution
(ii) ![]() → (a) intensive property
→ (a) intensive property
(iii) Κ → (b) depends on number of ions/volume
(iv) ΔrGcell → (c) extensive property
(i) Molar conductivity of the solution, Λm = ![]() = κ V
= κ V
(Electrodes are at unit distance)
Where, V= volume of the solution containing 1 mole of electrolyte.
In other words, molar conductivity of the solution is directly proportional to the volume of the solution. Thus, with dilution (increase in volume of the solution), Λm increases.
(ii) Intensive property is the property of the system that does not depend of the mass of the system.
![]() is an intensive property as it does not depend on the amount or size of the system. In fact, it depends on energy per coulomb of charge transferred.
is an intensive property as it does not depend on the amount or size of the system. In fact, it depends on energy per coulomb of charge transferred.
(iii) The conductivity or the specific conductance of electrolyte depends on the number of ions per unit volume.
(iv) ΔrGcell = -nFEcell
Where, ΔrGcell is the Gibbs free energy of the reaction.
F= Faraday constant
nF = Amount of charge passed
n= number of electron transferred in the radox reaction.
Since, ΔrGcell depends on the n, it is an extensive property.